Contraindications: AirDuo RespiClick is contraindicated in:
- Primary treatment of status asthmaticus or other acute episodes of asthma requiring intensive measures
- Patients with known severe hypersensitivity to milk proteins or any ingredients of AirDuo RespiClick
Serious Asthma-Related Events—Hospitalizations, Intubations, Death: Use of LABA as monotherapy (without an ICS) for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS (such as AirDuo RespiClick), data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone
Deterioration of Disease and Acute Episodes: AirDuo RespiClick should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of asthma. AirDuo RespiClick is not indicated for the relief of acute bronchospasm. An inhaled, short-acting beta2-agonist, not AirDuo RespiClick, should be used to relieve acute symptoms such as shortness of breath
Avoid Excessive Use and Avoid Use with Other Long acting Beta2-Agonists: AirDuo RespiClick should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medicines containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using AirDuo RespiClick should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason
Oropharyngeal Candidiasis has occurred in patients treated with AirDuo RespiClick. Advise patients to rinse the mouth with water without swallowing following inhalation
Immunosuppression and Risks of Infections: Patients who use corticosteroids, such as found in AirDuo RespiClick are at risk for potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. A more serious or even fatal course of chickenpox or measles may occur in susceptible patients. Use with caution in patients with the above because of the potential for worsening of these infections
Transferring Patients from Systemic Corticosteroid Therapy: Particular care is needed for patients who have been transferred from systemically active corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available ICS. Taper patients slowly from systemic corticosteroids if transferring to AirDuo RespiClick
Hypercorticism and Adrenal Suppression may occur with high doses of ICS, including fluticasone propionate, or at the recommended dose in susceptible individuals. If such changes occur, discontinue AirDuo RespiClick slowly
Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors: The use of strong cytochrome P450 3A4 (CYP3A4) inhibitors (e.g., ritonavir, ketoconazole) with AirDuo RespiClick is not recommended because increased systemic corticosteroid and increased cardiovascular adverse effects may occur
Paradoxical Bronchospasm and Upper Airway Symptoms: Paradoxical bronchospasm may occur. If bronchospasm occurs treat immediately with an inhaled short-acting bronchodilator, discontinue AirDuo RespiClick and institute alternative therapy
Hypersensitivity Reactions, Including Anaphylaxis: Immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, hypotension), including anaphylaxis, may occur after administration of AirDuo RespiClick. Discontinue AirDuo RespiClick if such reactions occur
Cardiovascular and Central Nervous System Effects: The salmeterol component of AirDuo RespiClick, can be associated with excessive beta-adrenergic stimulation which could present as the following symptoms: seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, palpitation, nausea, dizziness, fatigue, malaise, and insomnia. Use with caution in patients with cardiac arrhythmias, hypertension, coronary insufficiency. Drug may need to be discontinued in certain patients.
Reduction in Bone Mineral Density (BMD): Decreases in BMD have been observed with long-term administration of products containing ICS. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids) should be monitored and treated with established standards of care when using AirDuo RespiClick
Effect on Growth: ICS may cause a reduction in growth velocity. Patients should be maintained on the lowest dose of inhaled corticosteroid that effectively controls their asthma. Monitor growth of pediatric patients receiving AirDuo RespiClick.
Glaucoma and Cataracts: Long-term use of ICS, including fluticasone propionate, a component of AirDuo RespiClick, may increase the risk for cataracts or glaucoma. Regular eye exams should be considered
Eosinophilic Conditions and Churg-Strauss Syndrome: Systemic eosinophilic conditions, such as Churg-Strauss syndrome, may occur when using AirDuo RespiClick. Be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy
Coexisting Conditions: Use AirDuo RespiClick with caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, ketoacidosis, and in patients who are unusually responsive to sympathomimetic amines
Hypokalemia and Hyperglycemia: Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. Decrease in serum potassium are usually transient, not requiring supplementation. Be alert to hypokalemia and hyperglycemia in patient using AirDuo RespiClick
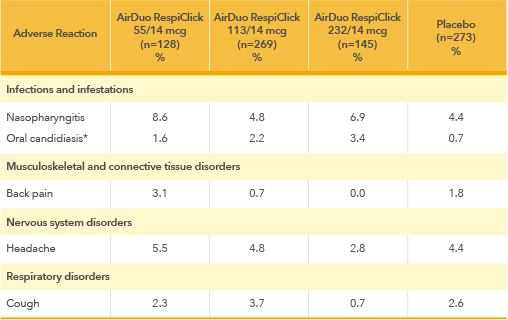
Adverse Reactions with AirDuo RespiClick: Most common adverse reactions (greater than or equal to 3%) include nasopharyngitis, oral candidiasis, headache, cough and back pain